The application shows the changes of thermodynamic state function in dependence of temperature and pressure by a reaction in tables and graphics
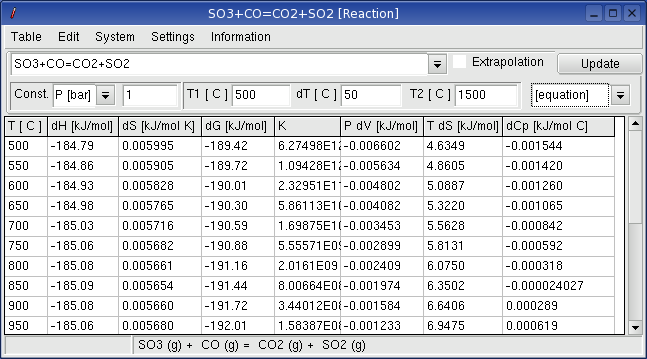
Enter a reaction equation in the table window and press the button update.
Reactants can be written with or without phase description, e.g.:
H2+1/2O2=H2O or
H2(g)+1/2O2=H2O(g)
When no any phase description is given, the application takes
the first dataset available in the database which is valid for the
given temperature. In case that no dataset exists for exactly the
required temperature, Reaction
takes the dataset with closest valid temperature range. The equation
for the reaction including the aggregate states is given in the status
line of the table.
For the reaction H2+1/2O2=H2O
The calculation will be carried out for H2O (l) if the first line of
the table shows 50 °C and 1 bar.
The calculation will be carried out for H2O (g) if the first line of
the table shows 300 °C and 1 bar.
The state functions can also be extrapolated if those data are not
available and extrapolation is admitted
Examples:
Fe2O3 = 2FeO+ 0.5 O2
Fe2O3=2FeO+ 1/2O2
Fe2O3=2Fe+3/2O2
Fe2O3=2Fe+1.5O2
3.2. Important
Menu
Functions
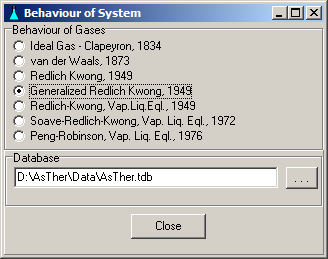
System -> behaviour: You can
determine, according to which behaviour the state functions of the
gases are to calculate. The state functions can be
calculated according to the real gases laws, when the critical data
(Tc,
Pc) of the gases are known in the database.
System ->
Select from Database:
The menu shows a window, in which substance of equation can be
selected.
The elements can be specified by Button Elements, which the
substances should contain.
When the buttons for the elements are pressed in the Elements window,
the colours will be changed to red or blue. The selected
elements are shown in red. When Button Accept is pressed, the
substances are shown in Substance
Window.
Mouse left double click on the Substance Window adds the selected
substance in the equation oft the the table window, or Button Accept
can be used.
|
|
|
System ->Graphic: Graphic Window usually shows
the
change of the state functions in depend of the temperature or pressure.
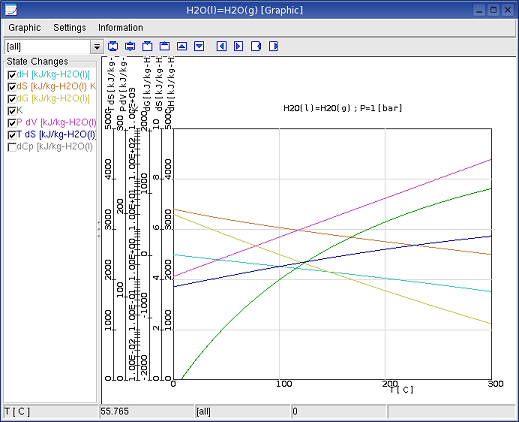
An other graphic variable can be defined by menu in Graphic Window
Graphic->User Defined
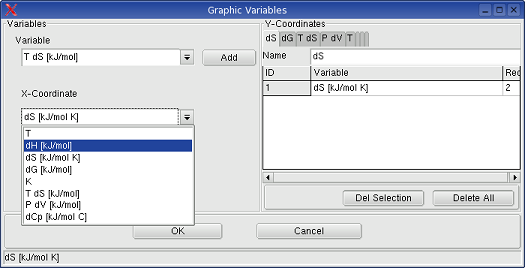
When Graphic->User Defined is selected, the
menu Graphic -> Variable definitions shows the following dialog box,
in which the graphic parameters can be selected individuelly.
More details are explained in 7.Graphic.
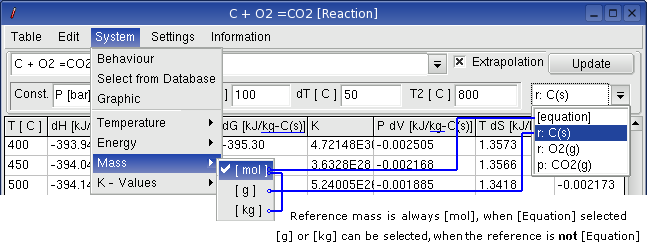
Dimension mass can be select, when a equation already calculated.
After the first calculation, the reactant can be specify by drag down
box (combo box) for reaction equation.
When you select a reactant or product of the reaction, the menus [g]
and [kg] can be enabled to select.
Information:
Information -> help:
displays this text.
Information -> messages:
recent error messages are listed in a separate window.
Information -> About Reaction:
information on software, version is displayed
3.2. Possible Errors
If the datasets of a substance contain gaps,
then calculations of the applications Pure Substance and Reaction can be erroneous.
Therefore, datasets of a substances should not contain any gaps.
For example: when two datasets exist for a substance in the database
first from 300-500 K and
second from 600-1000 K.
A calculation between 400 K and 700 K will not consider the second
dataset from 600-1000 K.